
Asia Pacific Journal of Family Medicine Volume 5 Issue 3
ORIGINAL ARTICLE
Impact of mood on the course of disease activity in patients with rheumatoid arthritis
Kiyomi SADAMOTO,1 Takehiko OGAWA,2 Takehisa OGURA2 and Eizo SAITO2
1The Department of Clinical Science, Toho University School of Pharmaceutical Sciences, Chiba and 2The Department of Rheumatology, Toho University, Ohashi Hospital, Tokyo, Japan
Correspondence: Kiyomi Sadamoto, 2-2-1 Miyama, Funabashi, Chiba, Japan.
Accepted for publication August 16, 2006.
Impact of mood on the course of disease activity in patients with rheumatoid arthritis
Kiyomi SADAMOTO,1 Takehiko OGAWA,2 Takehisa OGURA2 and Eizo SAITO2
1The Department of Clinical Science, Toho University School of Pharmaceutical Sciences, Chiba and 2The Department of Rheumatology, Toho University, Ohashi Hospital, Tokyo, Japan
Correspondence: Kiyomi Sadamoto, 2-2-1 Miyama, Funabashi, Chiba, Japan.
Accepted for publication August 16, 2006.
Abstract
Aims: This paper studies the influence of mood on the course of disease activity.
Methods: This prospective study collected 101 patients with rheumatoid arthritis (RA) who satisfied American College of Rheumatology criteria, and were stratified according to disease activity. The relationship between mood and activity was followed over 1 year.
Results: The better mood subgroup showed lower disease activity (serum C-reactive protein level and the number of affected joints) after 1 year as compared with the worse mood subgroup.
Conclusions: The present study demonstrated that mood itself influenced the course of disease activity of RA and the importance of mental care in RA therapy.
Key words: mood, rheumatoid arthritis, activity, course of disease, therapy
Methods: This prospective study collected 101 patients with rheumatoid arthritis (RA) who satisfied American College of Rheumatology criteria, and were stratified according to disease activity. The relationship between mood and activity was followed over 1 year.
Results: The better mood subgroup showed lower disease activity (serum C-reactive protein level and the number of affected joints) after 1 year as compared with the worse mood subgroup.
Conclusions: The present study demonstrated that mood itself influenced the course of disease activity of RA and the importance of mental care in RA therapy.
Key words: mood, rheumatoid arthritis, activity, course of disease, therapy
Introduction
Rheumatoid arthritis (RA) is a chronic disease with severe pain and deformity in many joints. Patients need to understand their disease and situation in any stage of RA. Even with new strategies in RA treatment, patients still need improvement in quality of life (QOL) in many aspects. Mood is one of the most important factors for QOL in RA patients in every day life. Several studies reported that mood itself may influence the immune-regulatory process,1–4 At the same time, several reports recently demonstrated that mood itself affects disease prognosis in diseases such as breast cancer, allergic dermatitis, herpes, hyperthyroidism and myocardial infarction.2–6 The aim of the present study was to clarify the influence of patients’ mood on the course of disease activity in RA.
Materials and methods
We collected questionnaires from 101 RA patients who satisfied the American College of Rheumatology (ACR) criteria of RA, of whom 84 were female, and 17 were male, with an average age of 54.9 years. RA activity of each patient was documented at the time of collection of the questionnaires and was prospectively evaluated after 1 year. The questionnaire used and reported in the previous paper, consisted of 43 items which include pain score, activities of daily living (ADL) scores, socio-economic status, working status and future prospects (see Appendix 1).7 Most of the items in the questionnaire were chosen from arthritis impact measurement scales (AIMS)8 which are common and reliable methods to analyze QOL in RA patients. We added the question of future prospects to this questionnaire. This item asks patients what life they will have after 1 year. For the most part of the questionnaire, we utilized the visual analog scale (VAS) to ensure with a simple check that patients answered precisely. Together with some other questionnaire items, patients’ moods were measured using the Lorish face scale (FS).9 FS has been accepted as a brief non-verbal scale which reflects moods of patients with not only RA, but also many other diseases, such as malignancy, and it is frequently used in clinical work. There are 20 faces in the scale, from a very happy (fine) face to a very sad face (1–20). Patients were asked their average mood during the previous month. C-reactive protein (CRP) serum levels, which is the most popular indicator of RA activity, and number of affected joints were used as the indicators of disease activity (normal limit of CRP < 0.3: Japanese ordinary measurement scale).
To reveal the influence of mood on the course of disease activity, RA patients were stratified depending on the RA activity. In each stratified group, patients were divided into two subgroups according to FS score (better FS subgroup [FS scores ≤ 10] and worse FS subgroups [FS scores ≥ 11]). Between the better FS subgroup and worse FS subgroup in each stratified group, there was no significant difference in male/female ratio, age and period of disease(Table 1). At the same time, by stratifying patients according to disease activity, we were able to render the initial levels of disease activity (CRP and number of affected joints) of both subgroups similar. Deterioration regarding number of affected joints was defined when this number increased by more than two joints and improvement was defined when the number decreased by more than two joints.
Data analysis and statistics
Student’s t-test and Chi-square test were used for statistical analysis. The alpha-value for significance was set at 0.05 for all tests performed. (Student’s t-test, Table 3, χ2, Table 4)
Results
Following the previous reports of QOL and mood of RA patients, we studied the relation of patients’ moods and RA activity. At the time of the first examination, we found a strong correlation between the FS scores and disease activity. Namely, patients with worse FS scores tended to manifest a higher disease activity than those with better FS scores (Table 2). However, among the patients with the same degree of disease activity, some patients had FS scores ≤ 10 (better FS subgroup), while some had FS scores ≥ 11 (worse FS subgroup). This means that there was difference of mood in the same activity groups (Table 2). In each stratified group according to CRP levels, the mean initial CRP level was not different between the better FS subgroup and the worse FS subgroup, but the mean of the CRP levels of the better FS subgroup was significantly lower than that of the worse FS subgroup after 1 year of observation (Table 3). Simultaneously, in each stratified group according to the number of affected joints, the better FS subgroup showed a higher ratio of improvement and lower ratio of deterioration after 1 year as compared with that of the worse FS subgroup (Table 4).
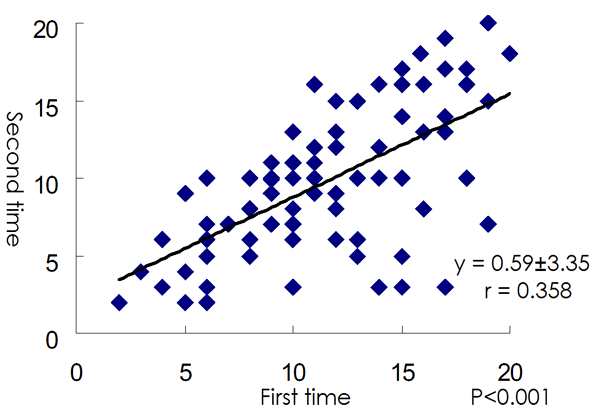
The better FS subgroup declared better future prospects than the worse subgroup. When patients were limited to those with elevated CRP levels, mean VAS scores of future prospects in the better mood subgroup were better than that in the worse mood subgroup (P < 0.01)(Table 5). Among patients with normal CRP levels, the same results were obtained (Table 5). The mean of future prospects in each subgroup did not change after 1 year. There was correlation between FS scores at the first year and those after 1 year (Fig. 1).
Discussion
Strong correlation between disease activity and mood was observed at the first examination. Patients with higher disease activity tended to manifest worse FS scores. Thus we thought it was necessary to avoid this bias of influence of disease activity on mood. To understand the influence of mood solely on the course of RA activity, we stratified the patients according to disease activity and prospectively compared prognosis of activity after 1 year between the better FS subgroup and the worse FS subgroup. Furthermore, stratifying patients according to disease activity rendered similar the effect of initial levels of disease activity on both subgroups. We inquired about patients’ average mood over the previous month. Mood may change in everyday life, but average mood seems to be constant to each individual.10–12 There was a correlation between FS scores at the first year and those after 1 year (Fig. 1). Each patient tends to declare a similar FS. There was strong relationship between FS and future prospects and this suggests that patients among the better mood subgroups seem to have more optimistic characters than those among worse mood subgroups. A previous study showed the relation of anxious mood and depression in patients with RA.13 The main subject of depression scores focuses on mood (Hamilton Depression Scale, Self-rating Depression Scale, DSM-IV). Thus, anxious mood among RA patients could be strongly influenced by patient personality.
The data from the present study demonstrated that patients with better mood showed lower disease activity after 1 year of observation as compared with those with worse mood. As well as several reports relating diseases and mood, mental status has also been reported to affect immuno-regulatoly systems.14–16 Chronic depression or chronic stress conditions lead to immuno-suppressive status and imbalance in corticotropin-releasing hormone, which induces cancer and hyperthyroidism.17 It has been reported that depressive states induce suppression of mitogenic reaction in lymphocytes, decreases the number and activity of natural killer cells, and decreases the production of interferon.16,18 Weigent also reported that in depressive states, specific receptors appear on the surface of immune-charged neuropeptide cells which induce productivity of cyclic AMP (cAMP) or cyclic guanosine monosphosphate (cGMP), and immuno-regulatory factors of tissue cells and is effected by second messengers.11 This could be related to the effect of mood on the course of activity of RA, which has been demonstrated in the present study. This is the first report to demonstrate the effect of mood on the course of disease activity among patients with RA. The results of this study show that the mood of RA patients requires attention to improve disease prognosis, and to improve patient mood is one of the most important targets in the treatment of RA.
Summary of implication for GPs
Arthritis is one of the common diseases in everyday clinical work. It is necessary to give an appropriate diagnosis for every patient, such as rheumatoid arthritis or osteoarthritis. To make the diagnosis, it is often useful to consult specialists in the early stages of the disease, after which GPs have the important role of maintaining their patient’s ADL and QOL, as well as prolonging their lifespan. This means that it is not only pain management that is important, but also integrated support, including monitoring patients’ mental conditions.
Acknowledgments
We thank all the patients and the people who cooperated with us in our research study.
References
1 Hirano D, Nagashima M, Ogawa R, Yoshino S. Serum levels of IL-6 and stress related substances indicate mental stress condition in patient with RA. J Rheumatol 2001; 28: 490–495.
2 Fujiwara R, Orita K. The enhancement of immune response by pain stimulation in mice. 1. The enhancement effect on PFC production via sympathetic nervous system in vivo and vitro. J Immunol 1987; 138: 3699–3703.
3 Laudeuslager ML, Ryan SM. Coping and immuno-suppression: inescapable but not escapable shock suppress lymphocyte proliferation. Science 1983; 221: 568–570.
4 Fukui Y, Sudo N, Yu XN, Nukina H, Sogawa H, Kubo C. The restraint stress-induced reduction in lymphocyte cell number in lymphoid organs correlates with the suppression of in vivo antibody production. J Neuroimmunol 1997; 79: 211–217.
5 Levy SM, Herberman RB, Lippman M, D’Angelo T, Lee J. Immunological and psychosocial predictors of disease recurrence in patients with early-stage breast cancer. Behav Med 1991; 17: 67–75.
6 Hotta M, Shibasaki T Masuda A, et al. The responses of plasma adrenocorticotropin and cortisol to corticotropin-releasing hormone (CRH) and cerebrospinal fluid immunoreactive CRH in anorexia nervosa patient. J Clin Endocrinol Metab 1986; 62: 319–324.
7 Sadamoto K, Fukuya H, Saito E. What is the factor that most influence QOL among Rheumatoid arthritis patients? Mod Rheumatol 2001; 11: 52–5.
8 Meenan RF. measuring health status in arthritis; The arthritis impact measurement scales. Arthritis Rheum 1980; 23: 146–52.
9 Lorish CD, Maisiak R. A breaf, nonverbal method for assessing patient mood. Arthritis Rheum 1986; 29: 906–9.
10 Hawley DJ. Sensitivity to change of the health assessment questionnaire (HAQ) and other clinical and health status measures in rheumatoid arthritis; Results of short-term clinical trials and observation studies versus long-term observation studies. Arthritis Care Res 1992; 5: 130–6.
11 Fries JF. Measurement of patient outcome in arthritis. Arthritis Rheum 1980; 23: 137–45.
12 Kiyomi S, Eizo S. Factors affecting on the mood among Japanese RA patients. APLAR J Rheumatol 2004; 7: 49–54.
13 Sharp L, Sensky T, Allard S. The course of depression in resent onset rheumatoid arthritis: the predictive role of disability, illness perception, pain and coping. J Psychosom Res 2001; 51: 713–9.
14 Weigent DA, Blalock JE. Interactions between the neuroendocrine and immune systems: common hormones and receptors. Immunological Rev 1987; 100: 79.
15 Bartrop RW, Luckhurst E, Lazarus L, Kiloh LG, Penny R. Depressed lymphocyte function after bereavement. Lancet 1977; I: 834–6.
16 Schleifer SJ, Keller SE, Camerino M, Thornton JC, Stein M. Suppression of lymphocyte stimulation following bereavement. J Am Med Assoc 1983; 250: 374–7.
17 Shekelle RB, Raynor WJ Jr, Ostfeld AM et al. Psychological depression and 17-year risk of death from cancer. Psychosom Med 1981; 43: 117–25.
18 Kiecolt-Glaser JK, Fisher LD, Ogrocki P, Stout JC, Speicher CE, Glaser R. Marital quality, marital disruption, and immune function. Psychosom Med 1987; 49: 13–34.
Table 1 - Comparison of sex, age and suffering period between better face scale (FS) subgroup and worse FS subgroup
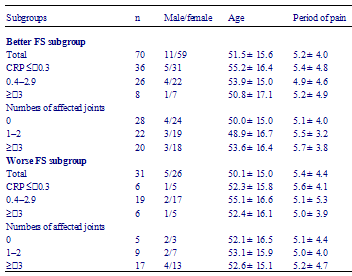
Table 2 - Frequency distribution of 101 patients with rheumatoid arthritis according to relation between face scale (FS) and disease activity at the time of first questionnaire (2002)
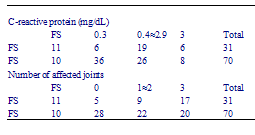
Table 3 - Comparison of C-reactive protein (CRP) levels after one year among better face scale (FS) group with those among worse FS group
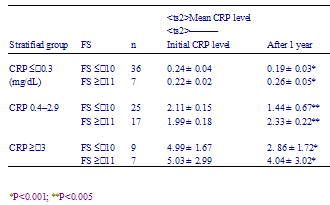
Table 4 - Comparison of number of affected joints after 1 years among better face scale (FS) group with those among worse FS group
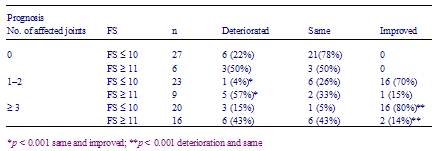
Table 5 - Future prospects in better face scale (FS) subgroup and worse FS subgroup in rheumatoid arthritis

Fig 1 - Scatter plot of Face Scale Score
Appendix 1 Questionnaire
1 How do you feel your physical stress in this month?
2 How frequently did you go out in this month?
3 Did you sleep well in this month?
4 Were you able to eat meal by yourself in this month?
5 Were you able to go to the toilet by yourself in this month?
6 Were you able to change cloth by yourself in this month?
7 Were you able to wash your face by yourself in this month?
8 Were you able to take a bath by yourself in this month?
9 Were you able to get into bed and go out from the bed by yourself in this month?
10 Have you tool exercise in this month?
11 Were you able to go up ladder by yourself in this month?
12 Were you able to walk by yourself in this month?
13 Were you able to carry baggage in this month?
14 Were you able to turn water tap in this month?
15 Were you able to squat down to get something in this month?
16 Were you able to write characters by yourself in this month?
17 Were you able to get on the bus and train by yourself in this month?
18 If you have family who live with you, who are they?
Grandfather, grandmother, father, mother, your spouse, children, grandchildren, others
19 How are your economic status?
20 How are the relationship with your friends?
21 Are you satisfied with your friends?
22 Do you take a public service for your disease?
23 Are you satisfied with a public service?
24 How amount of work (including domestic work) can you do?
25 Do your colleague and superior understand your disease?
26 Do you think mental problem affect your disease?
27 Do you think disease affect your mental condition?
28 Do you think how much disease affect your daily activities?
29 Do you think how disease affect your work?
30 How do you understand your disease?
a. I understand my disease very much.
b. I understand how to do against my disease.
c. I want to know more information about my disease and related matters.
31 What is your impression of your disease?
32 What degree of pain do you feel?
33 How long do you feel morning stiffness?
34 What is your most inconvenient thing?
35 What are your inconvenient things that come from disease?
36 What do you want to improve?
37 What do you think medical services that you are given?
38 How has been your disease in this year?
39 Do you have any accidents that affect your mental status in this year?
Yes. No.
40 Have you had any physical accidents in this year?
41 How will you be after one year?
42 What will you want for your medical service?
*VAS scales were used as the measurement of questionnaire except no. 18, 31, 40.
^top