Asia Pacific Journal of Family Medicine Volume 5 Issue 2
ORIGINAL ARTICLE
The Chronic Heart-failure Assistance by Telephone (CHAT) Study:
Assessment of telephone support for vulnerable patients with chronic disease
Julie YALLOP,1,5 Bianca CHAN,1 Leon PITERMAN,1 Andrew TONKIN,1,4 Andrew FORBES,1 Patricia M. DAVIDSON,2 Robyn CLARK,3 Elizabeth HALCOMB,2 Andrea NANGLE,4 Simon STEWART,3 Joanne CROUCHER1 and Henry KRUM,1. on behalf of the CHAT Study Group

Accepted for publication 19 July 2006.
The Chronic Heart-failure Assistance by Telephone (CHAT) Study:
Assessment of telephone support for vulnerable patients with chronic disease
Julie YALLOP,1,5 Bianca CHAN,1 Leon PITERMAN,1 Andrew TONKIN,1,4 Andrew FORBES,1 Patricia M. DAVIDSON,2 Robyn CLARK,3 Elizabeth HALCOMB,2 Andrea NANGLE,4 Simon STEWART,3 Joanne CROUCHER1 and Henry KRUM,1. on behalf of the CHAT Study Group
1Monash University, Melbourne, Victoria, 2University of Western Sydney and Western Sydney Area Health Service, Sydney, New South Wales, 3University of South Australia, Adelaide, South Australia, the 4National Heart Foundation of Australia, Melbourne, Victoria, Australia and 5University of Auckland, Auckland, New Zealand.
Correspondence: Julie Yallop, Department of Epidemiology and Preventive Medicine, Central and Eastern Clinical School, The Alfred, Commercial Road, Melbourne, Victoria 3004, Australia.Accepted for publication 19 July 2006.
Abstract
Aim: To determine whether telephone support using an evidence-based protocol for chronic heart failure (CHF) management will improve patient outcomes and will reduce hospital readmission rates in patients without access to hospital-based management programs.
Methods: The rationale and protocol for a cluster-design randomised controlled trial (RCT) of a semi-automated telephone intervention for the management of CHF, the Chronic Heart-failure Assistance by Telephone (CHAT) Study is described. Care is coordinated by trained cardiac nurses located in Heartline, the national call center of the National Heart Foundation of Australia in partnership with patients’ general practitioners (GPs).
Conclusions: The CHAT Study model represents a potentially cost-effective and accessible model for the Australian health system in caring for CHF patients in rural and remote areas. The system of care could also be readily adapted for a range of chronic diseases and health systems.
Key words: chronic disease management; chronic heart failure; integrated health care systems; nursing care, rural health services; telemedicine; telenursing
Methods: The rationale and protocol for a cluster-design randomised controlled trial (RCT) of a semi-automated telephone intervention for the management of CHF, the Chronic Heart-failure Assistance by Telephone (CHAT) Study is described. Care is coordinated by trained cardiac nurses located in Heartline, the national call center of the National Heart Foundation of Australia in partnership with patients’ general practitioners (GPs).
Conclusions: The CHAT Study model represents a potentially cost-effective and accessible model for the Australian health system in caring for CHF patients in rural and remote areas. The system of care could also be readily adapted for a range of chronic diseases and health systems.
Key words: chronic disease management; chronic heart failure; integrated health care systems; nursing care, rural health services; telemedicine; telenursing
Introduction
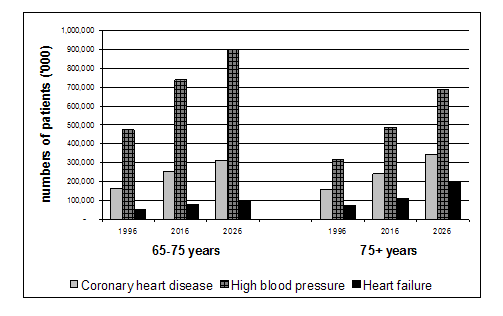
Chronic heart failure (CHF) is a major public health problem. It is associated with a worse prognosis than most cancers and requires frequent, prolonged, and costly admissions to hospital.1,2 The associated burden is particularly high in the elderly (Fig. 1).3 Increasing prevalence of CHF as well as the associated personal and economic burden mandate consideration and evaluation of new and innovative care models.4–6 As demonstrated by the recent DIAL trial undertaken in Argentina, clinical management augmented by specialist CHF nurses providing telephone support is one important approach and is clearly a system of care accessible to rural and remote patients.7–9
Materials and methods
Study design
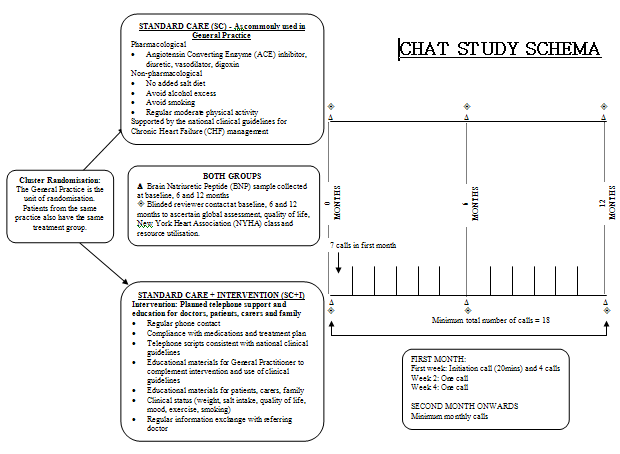
The chronic heart-failure assistance by telephone (CHAT) Study is a randomised trial of 534 people diagnosed with CHF in which all participants will receive standard care (SC) but half will receive a nurse-coordinated telephone intervention package (SC + I). This will use interactive voice response (IVR) software known as the TeleWatchTM telemedicine system developed by the Johns Hopkins University, USA.10 The nurse-coordinated telephone intervention package will be provided by experienced cardiac-trained registered nurses located in the National Heart Foundation of Australia call center – Heartline. A major component of the intervention package is in the form of prerecorded telephone CHF management scripts based on the Australian national guidelines for both the pharmacological and non-pharmacological management of CHF.11 The TeleWatchTM system is a telephone-based, semi-automated telemedicine system that enables a limited resource of health care providers, namely nurses, to monitor symptoms and support a larger than usual caseload of CHF patients.
Study aims and objectives
The primary aim of the CHAT study is to develop an effective preventative and supportive management strategy for Australians with CHF, particularly those residing in rural and remote communities. The study will test this aim by determining whether telephone support, involving outpatient education and monitoring using a multidisciplinary clinical protocol-based approach for the management of CHF will improve patients’ health status as represented by an innovative clinical composite score. Secondary aims include comparing total hospitalized days, the proportion of patients on target doses of angiotensin converting enzyme (ACE) inhibitors and changes in brain natriuretic peptide (BNP) levels. The cost-effectiveness of the intervention will also be determined.12
Study population
The study is currently recruiting Australia-wide with a target sample size of 534 CHF patients who are being predominantly cared for in rural and remote general practices as defined by Rural, Remote, Metropolitan Areas (RRMA) classification 3–7 and/or patients from RRMA classification 1 and 2 where access to formal CHF management programs is limited or inaccessible.13
General practititoners were self-selected for enrolment. Initially, the study was widely publicised to the Australian general practice population through various general practice networks and journals. GPs listed on national databases were sent a one-page ‘expression of interest’ fax-back letter of invitation. A member of the research team then contacted interested GPs regarding enrolment.
GP randomization
The general practice will be the unit of randomization. A cluster randomization trial is the design of choice aimed to prevent contamination between patient study groups. Once the GP has been assigned randomly to either the intervention or standard care study group, all patients recruited from that GP’s practice will automatically be assigned to the same study group. Participating practices will first be stratified by RRMA before being randomly allocated to the standard care or intervention group.
Patient recruitment
Appropriate to a cluster-design randomised controlled trial conducted in general practice, all patients from a single practice are assigned to the same study arm. The GP will act as guardian for their consenting patients in so far as their participating patients will consent without knowing to which study group their GP practice has already been allocated. Enrolling patients will only be given details of this assignment once informed consent has been provided and included only once the selection criteria have been met (Table 1). Randomised GPs will be asked to enrol between two and 10 CHF patients per practice.
Intervention vs. standard care
Intervention package for patients, their families and carers
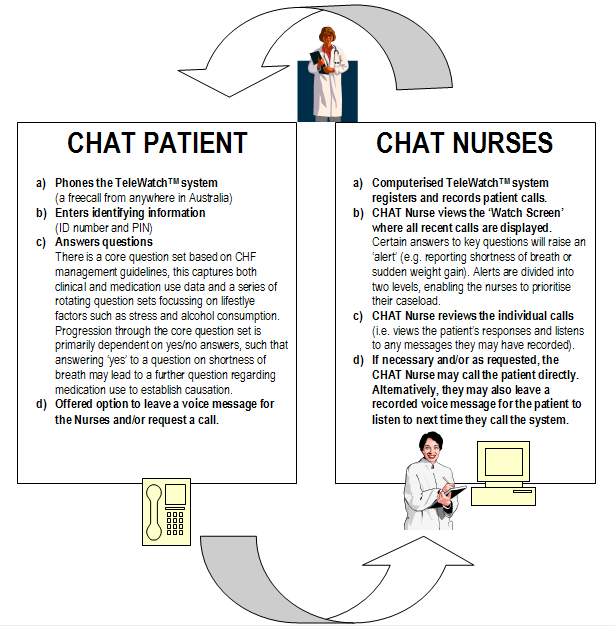
Half of the patient population will be randomly assigned to the intervention group and will receive a nurse-coordinated, CHF-focused intervention package. Each participant in this group will receive initial training in telephone calling from the ‘CHAT nurse’ to ensure competence in the independent utilization of the TeleWatchTM system technology. Once demonstrated, they will be instructed to call in, using a free-call 1800 number at no less than monthly intervals for a 12-month period (Fig. 2).
Responses entered by the patient are individually assessed by preprogramming of the TeleWatchTM system according to the patient’s overall health assessment and color-coded according to severity of signs and symptoms of worsening CHF (Fig. 3). The patient has the choice to leave a voice message for the nurse or to request a telephone follow-up call. The frequency of patient calls is monitored and recorded to allow for the exploration of a ‘dose effect’. We hypothesize a positive correlation between the frequency of calls and the effect of the intervention. Patients are more likely to learn skills associated with increased self-care activity the more feedback and reinforcement they receive through interacting with both the TeleWatchTM system and the CHAT nurses.
Participants and their families will be provided with an action plan on how to detect clinical deterioration and when and how to access emergency medical care and a personal copy of a Heart Foundation publication Let’s Talk about Heart Failure, prepared specifically for this study, and includes a lay-person’s version of the CHF national guidelines.14 They will also receive regular newsletters with helpful hints and information relating to their involvement in the study and will be given a individualized CHAT study patient diary to record all relevant clinical information, including visits to health services.
Intervention package for GPs
Each physician randomised to the intervention group will receive copies of the national guidelines for CHF management,11 and the ‘Diuretic Treatment Regimen’,15 a laminated double-sided desktop version of the CHF – Clinical Practice Guidelines for quick reference, ongoing targeted contemporary educational materials and regular study newsletters. The CHAT nurse will activate the GP fax-back feedback loop to report participants’ changing signs and symptoms to their GP (Fig. 4),16 and will activate a study-specific diuretic algorithm designed to maintain patients when they are not able to access their GPs.15
Standard care package for patients, their families and carers
General practitioners assigned to the standard care group will receive copies of the national guidelines for CHF management and the ‘Diuretic Treatment Regimen’.15,17 Their patients will receive an individualized CHAT Study patient diary but no other literature.
To assist with the development of the therapeutic relationship, reported as being more difficult where physical contact is precluded, personal and professional profiles including a photograph of the telephone support nurse and the independent evaluator working within the call center environment will be provided to all participants.
Patient follow-up
Participant contact will be at least monthly for a minimum of 12 months. The nurse-coordinated telephone intervention follow-up will be supported by specific CHF management training decision support software, the TeleWatchTM telemedicine system.10 Telephone surveys will be used to evaluate study clinical endpoints and will be administered at baseline, 6 months and 12 months by a trained independent interviewer who remains blinded with regard to patients’ treatment allocations. The survey will include questions relating to quality of life, economic assessment and utilization of health services.
Endpoint evaluations
The primary outcome variable will be a clinical composite (Packer) score,18 which combines changes in New York Heart Association class and the patient’s ‘global’ assessment. This latter measure asks the patient to judge whether their overall health status has changed since the commencement of the study and if so, to define the direction and estimate the magnitude of the change. Also included within the Packer composite score is the occurrence of major clinical events such as hospital admissions for or with CHF, all-cause mortality and withdrawal from intervention due to worsening heart failure within the study period.
Key secondary evaluations include total hospitalized days and patient quality of life (Table 2). Physiological evaluation of the intervention will be determined by BNP analysis. Both primary and secondary outcomes will also be compared in urban (RRMA 1–2) and rural and remote (RRMA 3–7) patients.
Data analysis and statistical methods
The primary outcome or Packer composite score is comprised of the following values: improved, no change or worsened. For example, assuming patients in the standard care arm would respond as 25% improved, 50% no change, 25% worsened, an odds ratio of 1.65 in a proportional odds model would improve the scores in the intervention arm to 36% improved, 48% no change, and 16% worsened. With patients individually randomised, to detect this change with 80% power would require 222 patients per arm. With an average of three patients per practice in a cluster-randomised design, an intrapractice correlation of 0.10, and applying the design effect for interval-scaled measures, the approximate sample size inflation factor is 20%, leading to a total sample size of 267 patients per arm.
Cost–effectiveness analysis
Cost-effectiveness will be assessed in a marginal analysis from a health system perspective, that is, including all private and public health service costs. The costs of the intervention and the cost offsets from reduced hospital admissions will be assessed from detailed data prospectively collected on drug utilization, investigations, hospital admissions, attendances at emergency departments and general practitioners (scheduled and unscheduled). This data will be supplemented using the Health Insurance Commission (HIC) and Department of Veterans’ Affairs (DVA).
The measure of health outcome will be derived from mortality outcomes and differences in quality of life (as measured by the Euroqol, a generic measure of health-related quality of life)19 and will be expressed in quality-adjusted life years (QALYs). The resultant cost-utility ratios (cost per QALY) can easily be redefined as a cost per disability adjusted life years (DALYs) because the disability weights used in the Australian Burden of Disease studies were derived for health states with a Euroqol description.20 All analyses will be on an intention-to-treat basis.
Discussion
Many clinical trials in CHF, whether of non-pharmacological or pharmacological therapies, including the recently published DIAL trial exclude up to 40% of the patients screened for the intervention on the basis of factors such as comorbid conditions and lack of proximity to the study center.21–23 Traditional empiricism demands homogeneity, but the reality is that in the community, CHF patients are heterogeneous.24,25 On the basis of these observations and a fundamental premise of the study which relates to the accessibility of care, in contrast to the DIAL trial, inclusion criteria have been designed to recruit a population that truly reflects ‘real world’ community practice, including patients with diastolic dysfunction. General practitioners will be recruited from practices identified by the RRMA to ensure the representative nature of study participants and will continue to provide ‘usual care’ during the 1-year study period. Recruitment will occur predominantly from RRMA classification 3–7 (rural and remote) general practices where access to formal CHF management programs is currently largely unavailable.
The inclusion of BNP as an objective measure will provide an opportunity to undertake a physiological evaluation of the intervention, which has not previously been evaluated in this manner, and to assess the prognostic capability of BNP within a CHF community-based patient population.
This is also the first study of its type where the participants are instructed to telephone the nurse rather than receive nurse-initiated telephone calls, which potentially could translate to care being provided for those in greatest need.
The CHAT study will also test a novel integrated and semiautomated IT support platform that enables patients to telephone as often as they choose, and at a time which suits them best. This automated service is aimed to help monitor patients’ progress and reinforce CHF self-care skills and activities while requiring fewer nurses than a traditional telephone-based health service.9
Acknowledgments
The CHAT investigators gratefully acknowledge the following funding sources: National Health and Medical Research Council of Australia, National Heart Foundation of Australia, Medical Benefits Fund of Australia, a Pfizer CVL Grant and My Chemist.
The CHAT Study Team would also like to acknowledge Dr Edward Kasper and Dr Jeff Spaeder, Department of Cardiology and Mr James Palmer, Applied Physics Laboratory Johns Hopkins University, Baltimore, Maryland, USA for the use of the TeleWatchTM telemedicine system along with ongoing expert advice and support of its usage.
Summary of implications for GPs
Compounding the pressure of a shrinking general practice (GP) workforce, Australia’s ageing population mandates the consideration and evaluation of new models of care. There is a growing expectation that GPs will play an increasing role in chronic disease management. The CHAT Study will test an innovative and affordable ‘system-of-care’ designed to support GPs and their patients with chronic heart failure (CHF). Many patients have limited access to formal CHF management programs, particularly in rural and remote areas. Through recruiting a population that truly reflects ‘real world’ community practice, this study will test the capacity of telenursing to support GPs, improve CHF patients’ quality of life and reduce their need for hospital readmission.
References
1 Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJ. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 2001; 3: 315–22.
2 McMurray JJ, Stewart S. Epidemiology, aetiology and prognosis of heart failure. Heart 2000; 83: 596–602.
3 Clark A, McLennon S, Dawson A, Wilkinson D, Stewart S. Uncovering a hidden epidemic. A Study of the Current Burden of Heart Failure in Australia. Heart Lung Circ 2004; 13: 266–76.
4 Riegel B, Carlson B, Glaser D, Hoagland P. Which patients with heart failure respond best to multidisciplinary disease management ? J Card Fail 2000; 6: 290–9.
5 McAlister F. Multidisciplinary Strategies for the Management of Heart Failure Patients at High Risk for Admission. J Am Coll Cardiol Foundation 2004; 4: 810–9.
6 Stewart S, Blue L, Walker A, Morrison C, McMurray JJ. An economic analysis of specialist heart failure nurse management in the UK; can we afford not to implement it? Eur Heart J 2002; 23: 1369–78.
7 GESICA Investigators. Randomised trial of telephone intervention in chronic heart failure: DIAL trial. BMJ 2005: 331: 425.
8 Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med 2002; 162: 705–12.
9 Nul DR. DIAL: Randomized Trial of Telephonic Intervention in Chronic Heart Failure. In: 75th Scientific Sessions of the American Heart Association, 2002. Chicago: American Heart Association, 2002.
10 TeleWatch telemedicine™, Master Manual, proprietary of the Johns Hopkins University (JHU) and the JHU Applied Physics Laboratory.
11 Krum H. National Heart Foundation of Australia and Cardiac Society of Australia & New Zealand Chronic Heart Failure Clinical Practice Guidelines Writing Panel. Guidelines for management of patients with chronic heart failure in Australia. Med J Aust 2001; 174; 459-66.
12 Richards AM, Nicholls MG, Yandle TG et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation 1998; 97: 1921–9.
13 Arundell L. Rural, Remote and Metropolitan Zones Classification: A Classification of Australia as at 30 June 1986 and A Methodology for 1991 Census Data. Canberra: Department of Primary Industries and Energy, 1991.
14 National Heart Foundation Australia. Let’s talk about heart failure: A guide for patients, their families and carers. Adelaide: NHF Australia, 2004.
15 Krum H. A Guideline for Flexible Diuretic Regimens. Melbourne: Monash University, 2004.
16 Clark R, Yallop J, Nangle A. GP Feedback Loop: Unpublished protocol, available from CHAT@chariot.net.au 2004.
17 Krum H, Tonkin AM, Currie R, Djundjek R, Johnston CI. Frequency, awareness and pharmacological management of chronic heart failure in Australian general practice. The cardiac awareness survey and evaluation (CASE) Study. Med J Aust 2001; 174: 439–44.
18 Packer M. Proposal for a new clinical end point to evaluate the efficacy of drugs and devices in the treatment of chronic heart failure. J Card Fail 2001; 7: 176–82.
19 The EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy (Amsterdam, Netherlands) 1990; 16: 199–208.
20 Mathers CD, Vos ET, Stevenson CE, Begg SJ. The Australian burden of disease study: Measuring the loss of health from diseases, injuries and risk factors. Med J Aust 2000; 172: 592–9.
21 Rich MW, Vinson JM, Sperry JC, et al. Prevention of readmission in elderly patients with congestive heart failure: Results of a prospective, randomised pilot study. J Gen Intern Med 1993; 8: 585–90.
22 Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive cardiac failure. New Engl J Med 1995; 333: 1130–95.
23 Naylor MD, Brooten D, Campbell R, Jacobsen BS, Mezey MD, Pauly MV. Comprehensive discharge planning for the hospitalised elderly. A randomised clinical trial. J Am Med Assoc 1999; 281: 613–20.
24 Ghali JK. The Cohere Registry. Hype of Hope. J Card Fail 2000; 6: 272–5.
25 Krum H, Tonkin AM, Currie R, Djundjek R, Johnston CI. Chronic heart failure in Australian general practice. The Cardiac Awareness Survey and Evaluation (CASE) Study. Med J Aust 2001; 174: 439–44.
26 Kelly DT. Our future society: a global challenge. Circulation 1997; 95: 2459–64.
Table 1 - Patient selection criteria
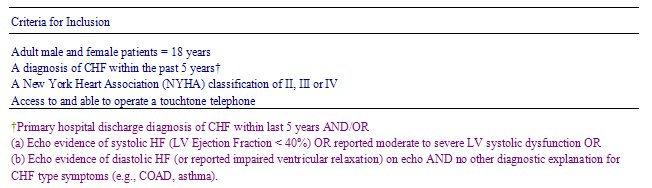
Table 2 - Outcome measures